HOW CAN WE HELP YOU? Call 1-800-TRY-CHOP
In This Section
Can Durability, Longevity of Bioprosthetic Heart Valves be Improved?
CHOP researchers discovered a way to modify bioprosthetic heart valves to prevent structural degeneration.
The findings:
Structural degeneration of bioprosthetic heart valves (BHV) can be prevented by modifying BHVs with a biocompatible, nonimmunogenic, and oxidation-resistant polymer, according to research from the Pediatric Heart Valve Center, part of a Frontier Program at Children's Hospital of Philadelphia. This study demonstrated that the use of poly-2-methyl-2-oxazoline (POZ) could make BHVs last longer, reducing patients' risk of reoperation.
Why it matters:
Heart valve disease, which typically involves the mitral or aortic valve, affects millions of people worldwide. With no effective medical therapy available, it is often treated with surgically implanted or transcatheter delivered bioprosthetic or mechanical valve prostheses. BHVs, which are fabricated from tissue grafts obtained from animal organs, such as bovine pericardium (BP), are the most frequently used valve replacements, as they are less likely to cause blood clots and do not require lifelong anticoagulation.
But structural valve deterioration (SVD) is common with BHVs. The reduced durability is caused by calcification and the accumulation of other recently recognized contributors: advanced glycation end products (AGE) and AGE-modified serum proteins. The limited longevity of the device often increases the risk for reoperation after only a decade or less of functionality, which is particularly significant for young patients.

Robert Levy, MD
Who conducted the study:
Pediatric Heart Valve Center researchers, including Senior Author Robert Levy, MD, director of Cardiology Research and the William J. Rashkind Endowed Chair in Pediatric Cardiology, as well as collaborators at the University of Pennsylvania and Columbia University conducted the study.
How they did it:
By exploring the option of modifying a BP-derived BHV with a polymer to increase its robustness, the researchers ultimately chose to study POZ, a polymer that shares similarities with polyethylene glycol (PEG) but does not have its propensity for both eliciting an immune response and degradation due to oxidization. POZ has been shown to prevent serum protein adsorption and mitigate cell adhesion to modified glass surfaces, but prior to this study, POZ had not been investigated in bioprosthetic heart valve formulations.
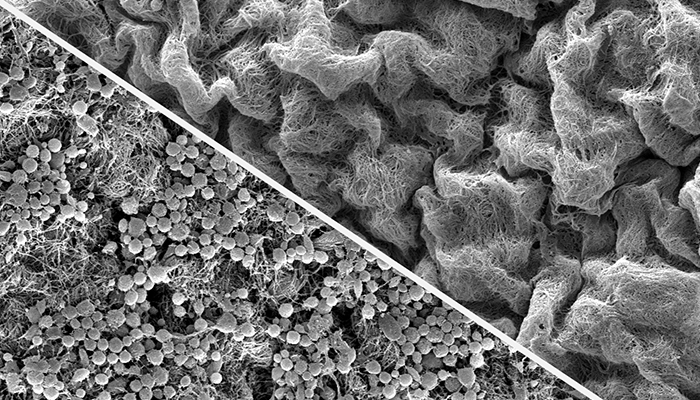
Extensive deposition of thrombus, formed elements of blood (red cells, white cells, platelets) as shown in the left image, was profoundly reduced if bioprosthetic leaflets were modified with POZ (right image).
The researchers showed not only could POZ be successfully incorporated into BHV biomaterials, but POZ also significantly reduced serum protein infiltration and AGE formation in vitro. Working with an animal model, they showed that POZ performed the same functions in vivo. Although the modified BHV did not prevent calcification, incubating the BP with ethanol, a Food and Drug Administration approved anti-calcification technology developed by Dr. Levy, followed by POZ modification before implantation significantly reduced calcification and AGE-serum protein accumulation. Also, the BHVs were more biocompatible than either unmodified counterparts or those modified with PEG.
Quick thoughts:
"These findings provide a potential strategy for preventing structural valve degeneration of bioprosthetic heart valves," Dr. Levy said. "The results of these investigations could be the basis for a novel approach that could improve the durability of these widely used devices and address a major unmet need."
Where the study was published:
The study appears in Proceedings of the National Academy of Sciences.
Want to learn more?
Read more in this CHOP press release.


