HOW CAN WE HELP YOU? Call 1-800-TRY-CHOP
In This Section
Enhancing the Effectiveness of NETs to Trap Bacteria
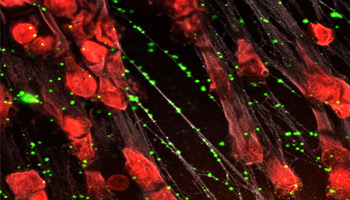
Neutrophil extracellular traps form weblike structures inside small blood vessels that immobilize microorganisms and reduce the potential for systemic infection.
droseyb [at] email.chop.edu (By Barbara Drosey)
Most of us recover from daily assaults on our immune systems, like when you’re cutting vegetables, the knife slips, and you accidentally slice your finger. Your body’s most important physical barrier — its skin and mucosal lining — against bacteria, microbes, and other infectious organisms is breached. That’s when neutrophils, the most abundant type of white blood cell, speed to the wound site to contain the spread of microbes, preventing a slight accident from becoming a life-threatening event.
Among neutrophils’ armamentarium is the release of neutrophil extracellular traps (NETs), the exploding nucleus of a neutrophil that forms a weblike structure inside of small blood vessels. This meshwork immobilizes microorganisms and reduces the potential for systemic infection, or sepsis, which accounts for one in five deaths around the world. While individuals undergoing cancer treatments or with conditions such as sickle cell anemia are more susceptible to sepsis due to their body’s weakened mechanisms for fighting infection, healthy people also may experience the ill effects of sepsis.
“Sepsis is a diffuse, prevalent disease that is underappreciated for how devastating it can be,” said Mortimer Poncz, MD, chief, Division of Hematology at Children’s Hospital of Philadelphia. “NETs have been recognized in the past 10 years as being part of the neutrophil response by binding to the bacteria and arresting it locally to prevent spread.”
Dr. Poncz and Kandace Gollomp, MD, also from the Division of Hematology, are lead principal investigators on grant awards from the National Heart, Lung, and Blood Institute to study new therapeutics that will enhance the body’s ability to ward off bacteria. Their study team will elucidate findings from their projects on an R35 and on a K99 mechanism to examine whether enhancing the stability of NETs to capture microbes could be a useful approach to prevent negative outcomes in sepsis.
The Role of Platelet Factor 4
While released NETs aid the body’s immune response, enzymes in the blood break these NETs down. A naturally occurring protein, platelet factor 4 (PF4), slows the disintegration process. PF4 sticks to long, negatively charged molecules like NETs, keeping them stable at the injury site and also being essential for the NETs to bind to a wide variety of bacteria much like a fly paper trap. Drs. Poncz and Gollomp first focused on the relationship between NETs and PF4 in the study of a thrombosis disorder called heparin-induced thrombocytopenia (HIT), but these studies led to insights that could enhance the role of NETs in sepsis.
“We found that NETs do not actually bind bacteria well,” Dr. Poncz said. “Since NETs and bacteria are both negatively charged and should naturally repel each other, we knew there had to be an intermediary involved. We showed that without the presence of PF4, there is minimal binding of bacteria to NETs.”
With this enhanced understanding, the study team hypothesized that administering additional PF4 would be beneficial in treating patients in the early stages of sepsis. Moreover, in their HIT studies, Drs. Poncz and Gollomp identified a monoclonal antibody that binds to PF4 when it is complexed to NETs called KKO.
“KKO cross-links PF4 on the NETs” Dr. Poncz explained. “This further protects the NETs from being digested in the blood by stabilizing their binding to PF4. In our animal models of sepsis, while treatment with PF4 alone offers some protection, infusion of KKO markedly enhances the protective effects of PF4, leading to striking improvements in survival.”
NIH Director Francis Collins, MD, PhD, took note of this NETs research, comparing the novel approach to creating stickier spider webs that entrap microbes at the site of breakdown in the skin.
“What we have at this point is a potential therapeutic for people, which is basically like Dr. Collins said — making the spider web better. By using the antibody, we are able to make sure we have as much PF4 bound to the spider web as possible and this allows us to greatly enhance the ability of the spider web to trap bacteria,” Dr. Poncz said.
The research team will move forward with studies into further improving outcomes in animal models of sepsis with the eventual goal of clinical application. Dr. Poncz foresees that in conjunction with CHOP’s algorithms in place to detect sepsis quickly, patients would receive a dose of the monoclonal antibody possibly alone or with added PF4 as an early therapeutic intervention.
The study team is eager to investigate the potential for widespread application in clinical settings using the antibody alone or in combination with PF4. They are also testing whether this same strategy would be effective at trapping viruses, including SARS-CoV-2 and flu, to prevent patients from developing severe outcomes as well.